As an ISO13485 certified and registered medical device manufacturer under the Japan law, we are rich in experience and knowledge of the entire medical device manufacturing process through own brand product manufacturing and launch. Those accumulated skills and expertise are fully utilized in order to meet and exceed customer outsourcing expectations.
Medical device production flow
Product planning
Development plan
Specification development
Basic design
Mass production prototyping
Application for Government Medical Regulations
Production
International support
Product Launch
Own brand products:Vascular screening device

| Equipment class | Class II |
|---|---|
| Medical device category | Diagnosis |
| Commissioned phase | Product planningDevelopment planSpecification developmentBasic designManufacturingOverseas (U.S.A) |
| Contents | Design and manufacture of in-house products, overseas expansion (U.S.A., Asia) |
Own brand products:Sensory measurement device
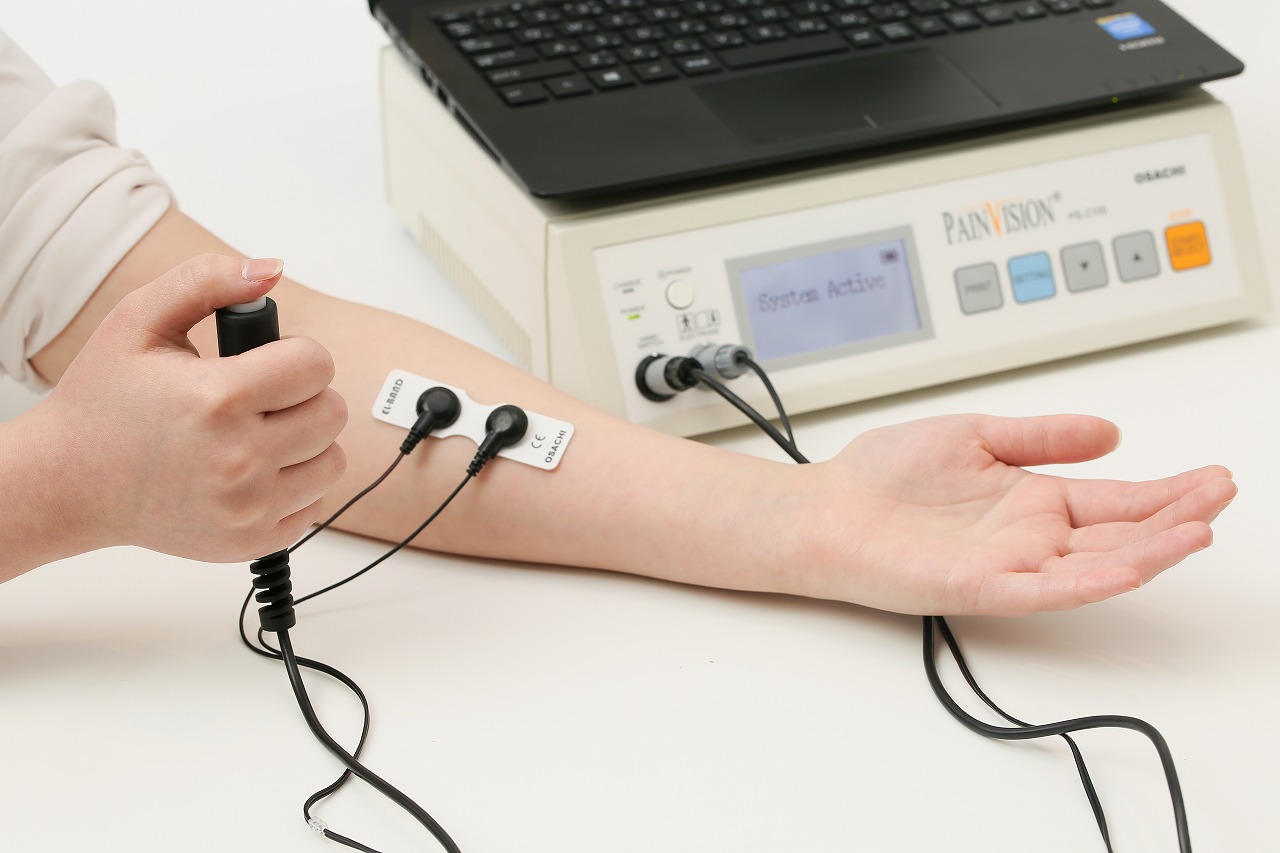
| Medical Device Class | Class II |
|---|---|
| Medical device category | Diagnosis |
| Worked phase | Product planningDevelopment planSpecification development Basic designManufacturing |
| Contents | Design and manufacture of in-house products |
Deliverable products:Blood flow measurement devices
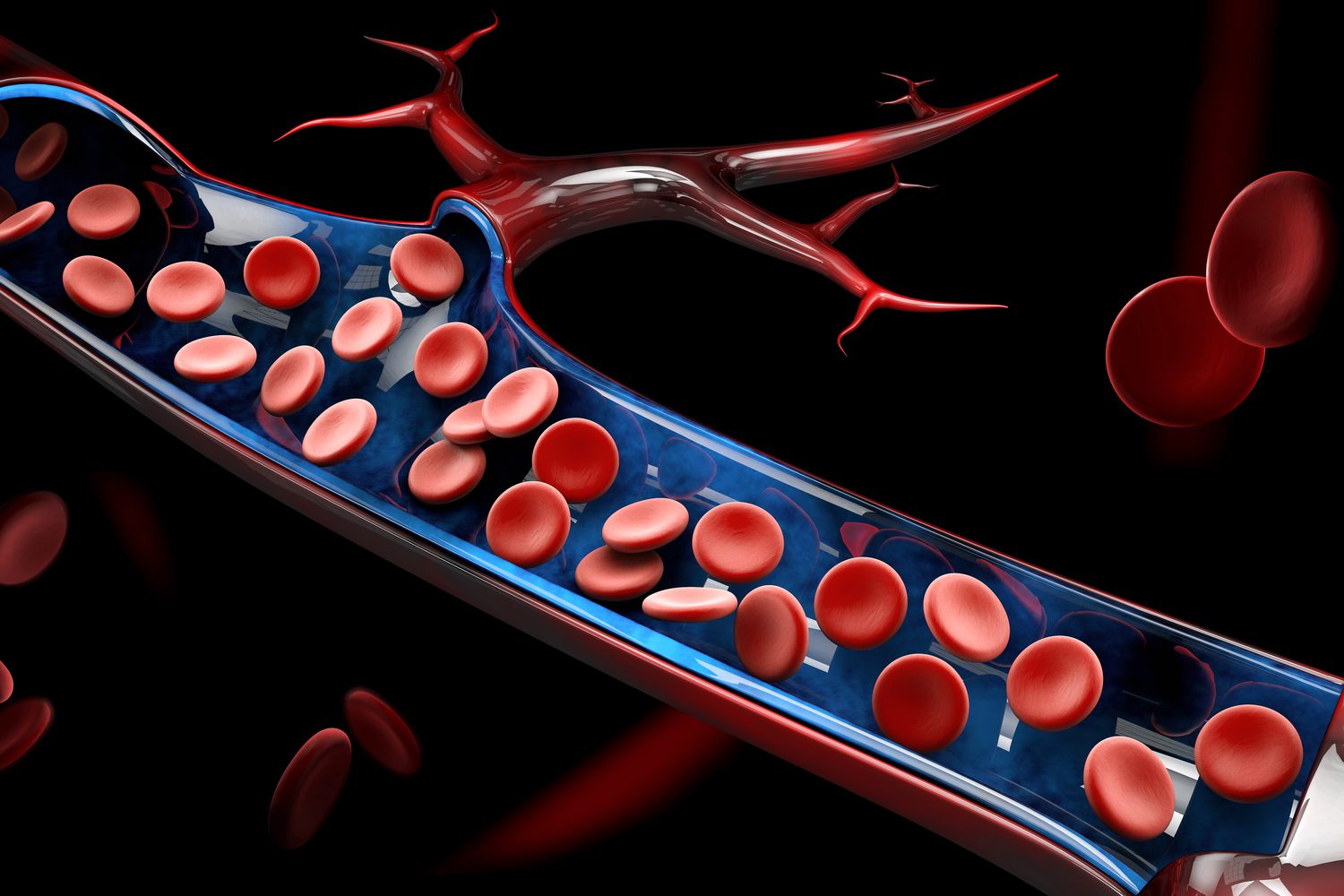
| Medical Device Class | Class II |
|---|---|
| Medical device category | Diagnosis |
| Worked phase | Specification developmentBasic designManufacturing |
| Contents | Design, prototyping, mass production |
Deliverable products:Surgical medical devices
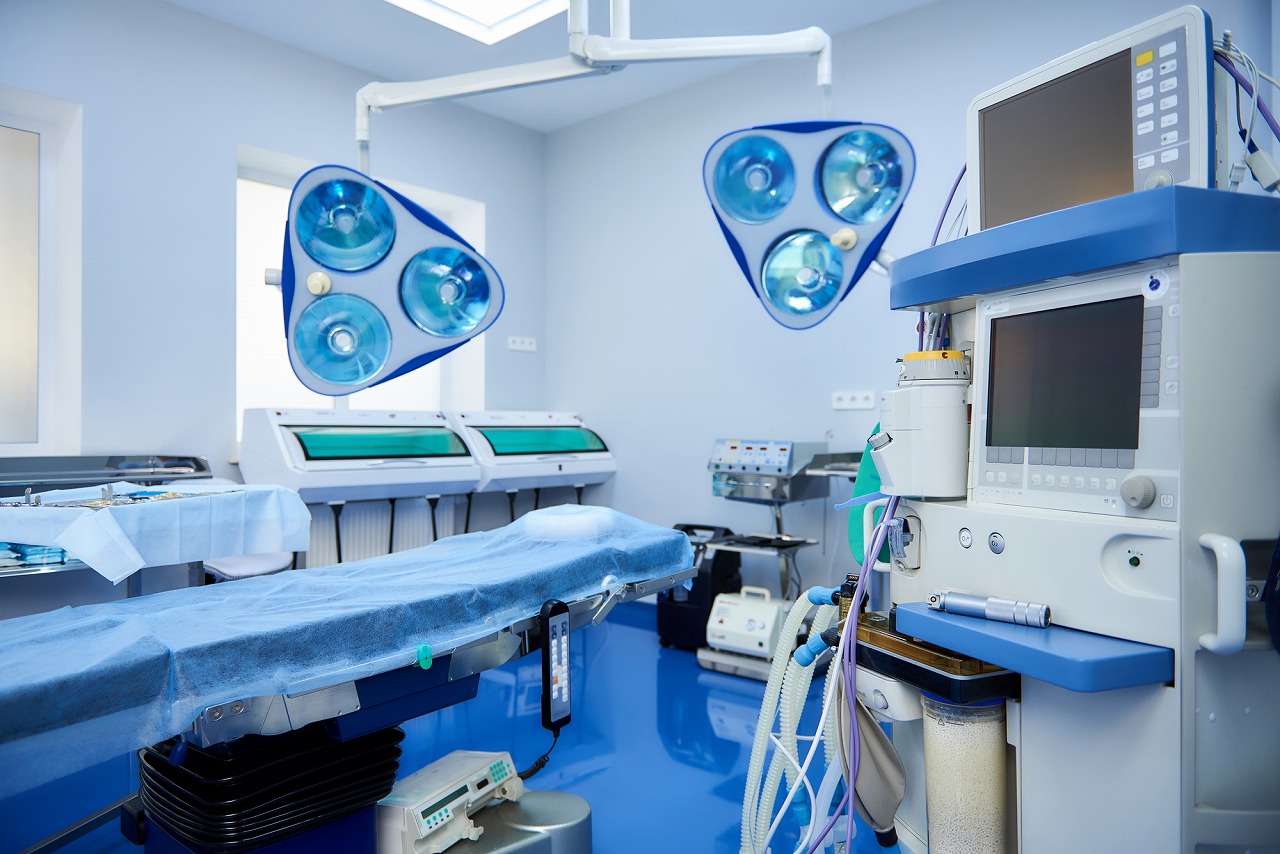
| Medical Device Class | Class II |
|---|---|
| Medical device category | Diagnosis |
| Worked phase | Development planSpecification developmentBasic designManufacturingOverseas (U.S.A.) |
| Contents | Design and manufacture of FDA-compatible medical devices |
Deliverable products:Metal cutting

| Medical device category | Others |
|---|---|
| Worked phase | Manufacturing (processing) |
| Contents | Orthopedic implants Surgical instruments High precision optical parts SUS630 Pure Titanium |
